- Home
- Therapeutic area
- Anti-infectives
Antibiotics, Appropriate antibiotics prescribing & Antimicrobial Stewardship
-
1. Overview of antibiotics
Antibiotics are natural substances produced by certain groups of microorganisms, and chemotherapeutic agents, which are chemically synthesized used in the treatment of disease.1
The modern era of antimicrobial chemotherapy is said to have began in 1929, with Fleming's discovery of the powerful bactericidal substance, penicillin, and Domagk's discovery in 1935 of synthetic chemicals (sulfonamides) with broad antimicrobial activity.1
Antibiotics may have a cidal (killing) effect or a static (inhibitory) effect on a range of microbes.1
Characteristics of Antibiotics 1
A clinically useful antibiotic should have as many of these characteristics as possible:
- It should have a wide spectrum of activity with the ability to destroy or inhibit many different species of pathogenic organisms.
- It should be nontoxic to the host and without undesirable side effects.
- It should be nonallergenic to the host.
- It should not eliminate the normal flora of the host.
- It should be able to reach the part of the human body where the infection is occurring.
- It should be inexpensive and easy to produce.
- It should be chemically stable (have a long shelf-life).
- Microbial resistance is uncommon and unlikely to develop.
An ideal antibiotic is an antibacterial agent that kills or inhibits the growth of all harmful bacteria in a host, regardless of site of infection without affecting beneficial gut microbes (gut flora) or causing undue toxicity to the host. Sadly, no such antibiotics exist. 2
Antibiotics Spectrum of action 1
- Broad spectrum: Antibiotics effective against procaryotes that kill or inhibit a wide range of Gram-positive and Gram-negative bacteria.
- Narrow spectrum: Antibiotics effective mainly against Gram-positive or Gram-negative bacteria.
- Limited spectrum: Antibiotics effective against a single organism.
Classes of antimicrobial agent and their mode of action 1
Chemical class Examples Biological source Spectrum (effective against) Mod of action Beta-lactams (penicillins and cephalosporins) Penicillin G, Cephalothin Penicillium notatum and
Cephalosporium speciesGram-positive bacteria Inhibits steps in cell wall (peptidoglycan) synthesis and murein assembly Semisynthetic beta-lactams Ampicillin, Amoxicillin Gram-positive and Gram-negative bacteria Inhibits steps in cell wall (peptidoglycan) synthesis and murein assembly Clavulanic Acid Amoxicillin plus clavulanic acid Streptomyces clavuligerus Gram-positive and Gram-negative bacteria Inhibitor of bacterial beta-lactamases Monobactams Aztreonam Chromobacterium violaceum Gram-positive and Gram-negative bacteria Inhibits steps in cell wall (peptidoglycan) synthesis and murein assembly Carboxypenems Imipenem Streptomyces cattleya Gram-positive and Gram-negative bacteria Inhibits steps in cell wall (peptidoglycan) synthesis and murein assembly Aminoglycosides Streptomycin Streptomyces griseus Gram-positive and Gram-negative bacteria Inhibits translation (protein synthesis) Aminoglycosides Gentamicin Micromonospora species Gram-positive and Gram-negative bacteria esp. Pseudomonas Inhibits translation (protein synthesis) Glycopeptides Vancomycin Amycolatopsis orientalisNocardia orientalis (formerly designated) Gram-positive bacteria, esp. Staphylococcus aureus Inhibits steps in murein (peptidoglycan) biosynthesis and assembly Lincomycins Clindamycin Streptomyces lincolnensis Gram-positive and Gram-negative bacteria esp. anaerobic Bacteroides Inhibits translation (protein synthesis) Macrolides Erythromycin, Azithromycin Streptomyces erythreus Gram-positive bacteria, Gram-negative bacteria not enterics, Neisseria, Legionella, Mycoplasma Inhibit translation (protein synthesis) Polypeptides Polymyxin Bacillus polymyxa Gram-negative bacteria Damages cytoplasmic membranes Polypeptides Bacitracin Bacillus subtilis Gram-positive bacteria Inhibits steps in murein (peptidoglycan) biosynthesis and assembly Polyenes Amphotericin Streptomyces nodosus Fungi (Histoplasma) Inactivate membranes containing sterols Polyenes Nystatin Streptomyces noursei Fungi (Candida) Inactivate membranes containing sterols Rifamycins Rifampicin Streptomyces mediterranei Gram-positive and Gram-negative bacteria, Mycobacterium tuberculosis Inhibits transcription (bacterial RNA polymerase) Tetracyclines Tetracycline Streptomyces species Gram-positive and Gram-negative bacteria, Rickettsias Inhibit translation (protein synthesis) Semisynthetic tetracycline Doxycycline Gram-positive and Gram-negative bacteria, Rickettsias Ehrlichia,
BorreliaInhibit translation (protein synthesis) Chloramphenicol Chloramphenicol Streptomyces venezuelae Gram-positive and Gram-negative bacteria Inhibits translation (protein synthesis) Quinolones Nalidixic acid synthetic Mainly Gram-negative bacteria Inhibits DNA replication Fluoroquinolones Ciprofloxacin synthetic Gram-negative and some Gram-positive bacteria (Bacillus anthracis) Inhibits DNA replication Growth factor analogs Sulfanilamide, Gantrisin, Trimethoprim synthetic Gram-positive and Gram-negative bacteria Inhibits folic acid metabolism (anti-folate) Growth factor analogs Isoniazid (INH) synthetic Mycobacterium tuberculosis Inhibits mycolic acid synthesis; analog of pyridoxine (Vit B6) Growth factor analogs para-aminosalicylic acid (PAS) synthetic Mycobacterium tuberculosis Anti-folate -
2. Rational Use of Antibiotics
A systematic review showed that inappropriate use of antibiotics was common especially in the developing countries with poor health care systems. 3 Inappropriate use of antibiotics can result in bacteria being resistant to antibiotics in the community. The acceleration of antibiotic resistance and the decline in the development of new antibiotics to combat the problem has created significant public health challenges to health policy makers, health care workers, and the population around the world.4
According to the WHO, Inappropriate/irrational use of antibiotics in humans and agriculture is one of the drivers of the emergence of antimicrobial resistance.5
Medicine use is said to be rational (appropriate, proper, correct) when patients receive the appropriate medicines, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost both to them and the community. Irrational (inappropriate, improper, incorrect) use of medicines is when one or more of these conditions is not met.6
Use of medicines is a critical factor in health system efficiency. The spend on medicine accounts for up to a fifth of all health spending, or even more in some countries. However, medicines are often overused (e.g. antibiotics) or underused (e.g. non‐adherence), resulting in avoidable adverse events and poor health outcomes.6
Irrational use of medicines is a major problem worldwide. World Health Organization (WHO) estimates that more than half of all medicines are prescribed, dispensed or sold inappropriately, and that half of all patients fail to take them correctly. The overuse, underuse or misuse of medicines results in wastage of scarce resources and widespread health hazards.7
Examples of irrational use of medicines include: use of too many medicines per patient ("poly-pharmacy"); inappropriate use of antimicrobials, often in inadequate dosage, for non-bacterial infections; over-use of injections when oral formulations would be more appropriate; failure to prescribe in accordance with clinical guidelines; inappropriate self-medication, often of prescription-only medicines; non-adherence to dosing regimens.7
World Health Organization (WHO) estimated that 80% of antibiotics is used in the community, of which about 20–50% is used inappropriately. As a result, WHO recommends involvement of the community in tackling of antibiotic resistance through improving access to medical services, reducing unnecessary use of antibiotics, taking a full course of treatment, not sharing medications with other people, and not keeping part of the course for another occasion.4
WHO advocates 12 key interventions to promote more rational use 7
- Establishment of a multidisciplinary national body to coordinate policies on medicine use
- Use of clinical guidelines
- Development and use of national essential medicines list
- Establishment of drug and therapeutics committees in districts and hospitals
- Inclusion of problem-based pharmacotherapy training in undergraduate curricula
- Continuing in-service medical education as a licensure requirement
- Supervision, audit and feedback
- Use of independent information on medicines
- Public education about medicines
- Avoidance of perverse financial incentives
- Use of appropriate and enforced regulation
- Sufficient government expenditure to ensure availability of medicines and staff.
-
3. Antimicrobial Resistance
Antimicrobial resistance (AMR) is the ability of a microorganism (like bacteria, viruses, and some parasites) to stop an antimicrobial (such as antibiotics, antivirals and antimalarials) from working against it. As a result, standard treatments become ineffective, infections persist and may spread to others.8
Antimicrobial resistance (AMR) including multidrug resistance (MDR), is on the rise among many microorganisms in health-care facilities as well as in community.9
Antimicrobial resistance (AMR) threatens the effective prevention and treatment of an ever-increasing range of infections caused by bacteria, parasites, viruses and fungi.
Antimicrobial resistance happens when microorganisms (such as bacteria, fungi, viruses, and parasites) change when they are exposed to antimicrobial drugs (such as antibiotics, antifungals, antivirals, antimalarials, and anthelmintic).10
Unless antibiotic resistance problems are detected as they emerge, and actions are taken immediately to contain them, society could be faced with previously treatable diseases that have become again untreatable, as in the days before antibiotics were developed.11
In retrospect, it is not surprising that resistance to penicillin in some strains of staphylococci was recognized almost immediately after introduction of the drug in 1946. Likewise, very soon after their introduction in the late 1940s, resistance to streptomycin, chloramphenicol and tetracycline was noted. By 1953, during a Shigella outbreak in Japan, a strain of the dysentery bacillus (Shigella dysenteriae) was isolated which was multiple drug resistant, exhibiting resistance to chloramphenicol, tetracycline, streptomycin and the sulfonamides. Over the years and continuing into the present almost every known bacterial pathogen has developed resistance to one or more antibiotics in clinical use.11
Causes of AMR
The causes of antimicrobial resistance (AMR) in developing countries are complex and may be rooted in practices of health care professionals and patients’ behavior towards the use of antimicrobials as well as supply chains of antimicrobials in the population. Some of these factors may include inappropriate prescription practices, inadequate patient education, limited diagnostic facilities, unauthorized sale of antimicrobials, lack of appropriate functioning drug regulatory mechanisms, and non-human use of antimicrobials such as in animal production.12
Multiple drug resistant organisms 11
Multiple drug resistant organisms are resistant to treatment with several, often unrelated, antimicrobial agents as described above in Shigella. Some of the most important types of multiple drug resistant organisms that have been encountered include:
- MRSA - methicillin/oxacillin-resistant Staphylococcus aureus
- VRE - vancomycin-resistant enterococci
- ESBLs - extended-spectrum beta-lactamases (which are resistant to cephalosporins and monobactams)
- PRSP - penicillin-resistant Streptococcus pneumoniae
Global burden of antimicrobial resistance
According to the O’Neil report, AMR kills an estimated 700,000 people worldwide each year, a number that could rise to 10 million by 2050 (which is estimated to be more than cancer deaths) if the problem is left unresolved.13
Deaths attributable to AMR worldwide every year compared to other major causes of death 13
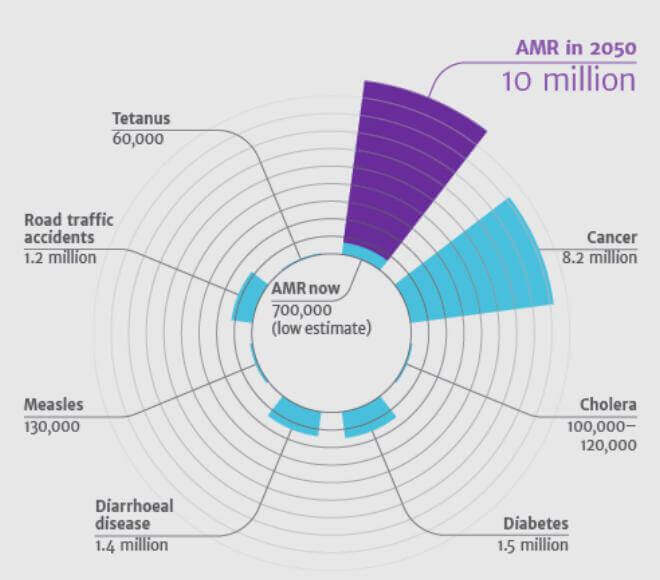
Source: O’Neill J. Antimicrobial resistance Currently, there are new emerging antibiotic-resistant bacteria trend, with about 70% resistant to at least one of the drugs most commonly used in treating infections.14
Combating AMR
A multidimensional approach (Figure below) is recommended to prevent AMR in a healthcare setting.14
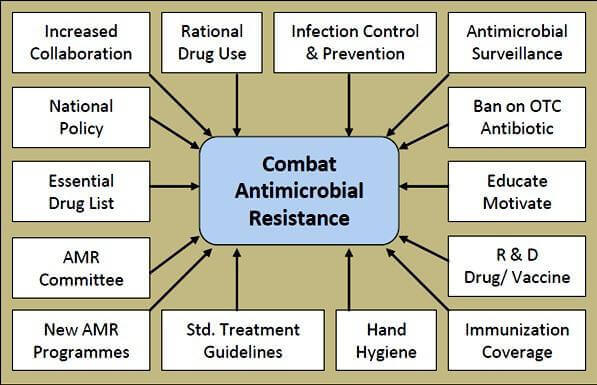
Source: O’Neill J. Antimicrobial resistance The Centers for Diseases Control and prevention (CDC) developed a campaign aimed at clinicians to prevent AMR. The campaign, entitled "Prevent Antimicrobial Resistance," centers around four key strategies for preventing antimicrobial resistance in healthcare settings:14
- Preventing infection,
- Diagnosing and treating infection effectively,
- Using antimicrobial wisely, and
- Preventing transmission of drug-resistant pathogens.
Within these strategies are 12 specific action steps derived from evidenced-based guidelines.15
Action Step 1: Vaccinate
- Get influenza vaccine
- Give influenza / S. pneumonia vaccine to at-risk patients before discharge
Action Step 2: Get the catheters out
- Use catheters only when essential
- Remove catheters when no longer essential
Action Step 3: Target the pathogen
- Culture the patient
- target empiric therapy to likely pathogens
- target definitive therapy to known pathogens
Action Step 4: Access the experts
- consult infectious diseases experts for patients with serious infections
Action Step 5: Practice antimicrobial control
- engage in local antimicrobial control efforts
Action Step 6: Use local data
- know your antibiogram
Action Step 7: Treat infection, not contamination
Action Step 8: Treat infection, not colonization
Action Step 9: Know when to say "no" to vanco(mycin)
Action Step 10: Stop antimicrobial treatment
- when infection is treated or unlikely
Action Step 11: Isolate the pathogen
- use standard infection control precautions
- contain infectious body fluids (airborne/droplet/contact precautions)
- when in doubt, consult infection control experts
Action Step 12: Break the chain of contagion
- stay home when you are sick
- keep your hands clean
- set an example!
-
4. Antimicrobial Stewardship Programmes
Antimicrobial Stewardship is defined by the WHO as a coherent set of actions which promote the responsible use of antimicrobials. This definition can be applied to actions at the individual level as well as the national and global level, and across human health, animal health and the environment.16
Antimicrobial stewardship programme (AMS programme): is defined as an organizational or system-wide health-care strategy to promote appropriate use of antimicrobials through the implementation of evidence-based interventions.16
The aim of an AMS programme is 16
- to optimize the use of antibiotics;
- to promote behaviour change in antibiotic prescribing and dispensing practices;
- to improve quality of care and patient outcomes;
- to save on unnecessary health-care costs;
- to reduce further emergence, selection and spread of AMR;
- to prolong the lifespan of existing antibiotics;
- to limit the adverse economic impact of AMR; and
- to build the best-practices capacity of health-care professionals regarding the rational use of antibiotics.
CDC 7 Core Elements of Hospital Antibiotic Stewardship Programs 17

Hospital Leadership Commitment Dedicate necessary human, financial, and information, technology resources.

Accountability Appoint a leader or co-leaders, such as physician and pharmacist, responsible for program management and outcomes.
Pharmacy Expertise (previously "Drug Expertise') Appoint a pharmacist, ideally as the co-leader of the stewardship program, to help lead implementation efforts to improve antibiotics use.

Action Implement interventions, such as prospective audit and feedback or preauthorization, to improve antibiotic use.

Tracking Monitor antibiotic prescribing, impact of interventions, and other important outcomes, like C. difficile infections and resistant patterns.

Reporting Regularly report information on antibiotic use and resistance to prescribers, pharmacists, nurses, and hospital leadership.

Education Educate prescribers, pharmacists, nurses, and patients about adverse reactions from antibiotics, antibiotic resistance, and optimal prescribing.
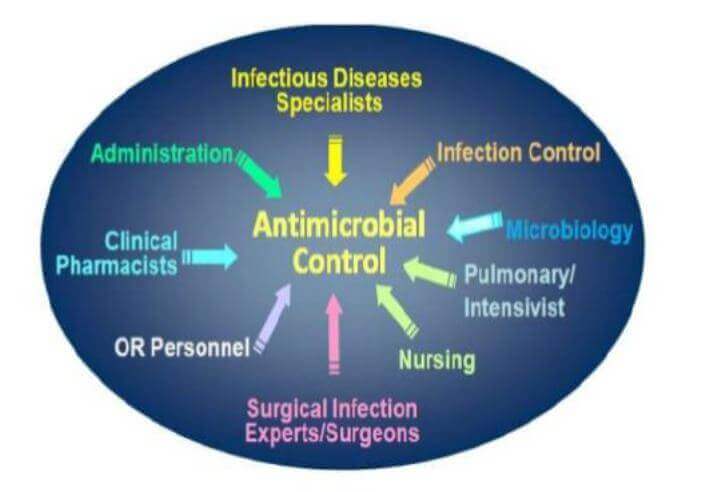
Antimicrobial Stewardship Committees/Teams
This AMS committee can be either stand-alone or embedded in another existing committee structure (e.g. drug and therapeutics committee, pharmacy committee, infection control committee, patient safety committee). If embedded in another committee, AMS must be a standing item on the committee’s agenda. The AMS committee is explicitly in charge of setting and coordinating the AMS programme/strategy according to its terms of reference.16
Antimicrobial Stewardship Team 18
-
5. References
- Kenneth Todar, PhD, Antimicrobial Agents in the Treatment of Infectious Disease, Todar’s online textbook of bacteriology, www.textbookofbacteriology.net
- Singh SB, Young K, Silver LL. What is an "ideal" antibiotic? Discovery challenges and path forward. Biochemical Pharmacology, 10 Jan 2017, 133:63-73
- Kardas P, Devine S, Golembesky A, Roberts C. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. International journal of antimicrobial agents. 2005;26(2):106–13. Epub 2005/07/13. 10.1016/j.ijantimicag.2005.04.017
- Gebeyehu E, Bantie L, Azage M. Inappropriate Use of Antibiotics and Its Associated Factors among Urban and Rural Communities of Bahir Dar City Administration, Northwest Ethiopia. PLoS One. 2015;10(9):e0138179. Published 2015 Sep 17. doi:10.1371/journal.pone.0138179
- Tangcharoensathien V. Complex determinants of inappropriate use of antibiotics. Bulletin of the World Health Organization 2018;96:141-144.
- World Health Organization (WHO). The pursuit of rational use of medicines: sharing and learning from country experiences, 2012 Available at https://apps.who.int/iris/bitstream/handle/10665/75828/WHO_EMP_MAR_2012.3_eng.pdf;jsessionid=11429ED27728648B682FF79D4CA775D2?sequence=1 Accessed June 28, 2020
- World Health Organization (WHO). Essential medicines and health products. Available at: https://www.who.int/medicines/areas/rational_use/en/ Accessed June 28, 2020
- Antimicrobial resistance, WHO Newsletter No.32; Nov 2017. Available at: http://www.who.int/antimicrobial-resistance/en/ Accessed on June 2020
- Uchil RR, Kohli GS, Katekhaye VM, Swami OC. Strategies to combat antimicrobial resistance. J Clin Diagn Res. 2014;8(7):ME01-ME4. doi:10.7860/JCDR/2014/8925.4529
- World Health Organization (WHO). Antimicrobial resistance. Available at: https://www.who.int/health-topics/antimicrobial-resistance June 28, 2020
- Kenneth Todar, PhD, Bacterial Resistance to Antibiotics, Todar’s online textbook of bacteriology, www.textbookofbacteriology.net
- Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017; 6:47. doi:10.1186/s13756-017-0208-x
- O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Review on antimicrobial resistance. 2014. Available at: https://amr-review.org/sites/default/files/AMR Review Paper - Tackling a crisis for the health and wealth of nations_1.pdf Accessed June 2020
- Awosan K J, Ibitoye P K, Abubakar A K. Knowledge, risk perception and practices related to antibiotic resistance among patent medicine vendors in Sokoto metropolis, Nigeria. Niger J Clin Pract 2018;21:1476-83
- CDC Campaign to Prevent Antimicrobial Resistance in Healthcare Settings, 12 Steps to Prevent Antimicrobial Resistance among Long-term Care Residents. Department of Health and Human Services Centers for Disease Control and Prevention. March 2004. Available at: https://www.cdc.gov/media/pressrel/r020326.htm Accessed June 28, 2020
- World Health Organization (WHO) Antimicrobial Stewardship Programmes in Healthcare Facilities in Low- and Middle-Income Countries: A WHO PRACTICAL TOOLKIT 2019. Available at: https://apps.who.int/iris/handle/10665/329404 Accessed June 28, 2020
- CDC. The Core Elements of Hospital Antibiotic Stewardship Programs: 2019. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf Accessed June 28, 2020
- Antibiotic stewardship program in children, Available at: https://images.app.goo.gl/QWER485S3gL9qWTu6 June 28, 2020
MAT-GH-2000028 | August 2020
